The water molecule is a chemical compound of hydrogen and oxygen, with the chemical formula H2O (consisting of two hydrogen atoms and one oxygen atom).
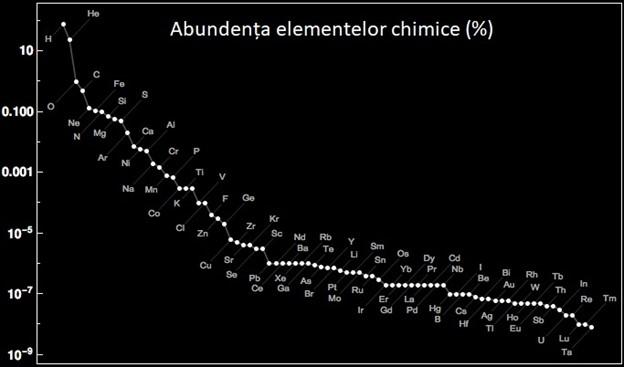
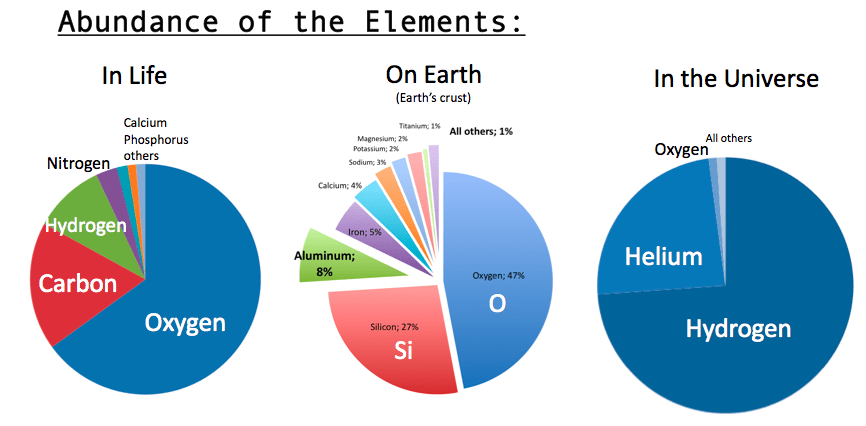
Hydrogen, the first chemical element needed to obtain water, is the simplest chemical element (it has atomic number 1, so it consists of a single proton) and is the most widespread in the universe. Hydrogen together with helium (atomic number 2) make up 98% of the matter in the universe (75% hydrogen and 23% helium).
Life on Earth has adapted to breathe oxygen. Although pure oxygen is poisonous, organisms have learned to use it in various chemical reactions necessary for metabolism. Oxygen favors combustion and if the atmosphere did not contain so much nitrogen, then any spark would have caused the whole Earth to be engulfed in flames. Oxygen is the third most abundant chemical element in the universe (about 1%), after hydrogen and helium. All other chemical elements in the periodic table make up the remaining 1%.
We can create pure water by combining hydrogen with oxygen, 2H2 = O2 -> 2H2O. But beware, this reaction is spontaneous and explosive.
Many other reactions can be used to produce water. Water can also be produced by burning hydrocarbons (eg methane or octane). The combustion of hydrocarbons will also produce water, among other chemical compounds. Another example is the reaction between baking soda and vinegar. The reaction produces carbonic acid (H2CO3) which decomposes rapidly into carbon dioxide and water.
Water can also be obtained by neutralization (a type of chemical reaction in which an acid reacts quantitatively with a base).
If the ingredients needed to get water are so abundant, why is water so rare in the universe? Or it isn’t?
Necessary ingredients
Mixing the two gases is not enough to start the reaction. In order for hydrogen gas and oxygen to react to form water, we need to add a certain amount of energy to the system to start the reaction, and this energy is called ‘activation energy’. A good source of energy could be an open flame. Once the reaction has begun, the energy emitted during the exothermic reaction is sufficient to create a chain reaction.
In ordinary chemical reactions (as opposed to nuclear reactions), the law of conservation of mass applies, ie the mass of the products is equal to the mass of the reactants. If we take hydrogen (H2) and oxygen (O2) to form water, then the chemical reaction is as follows: 2H2 (g) + O2 (g) -> 2H2O (g). If we calculate the mass for the reactants and for the products for this reaction, the mass will be equal. 3 moles of gas (2 moles of H2 + 1 mol of O2) react to form 2 moles of gas (2 moles of H2O). The mass is equal, but the volume is low (especially after the water condenses into a liquid). In this reaction almost all hydrogen and oxygen react very quickly to form water vapor.
Although H2O cannot exist in stars, hydrogen and oxygen can exist in them separately. Hydrogen is the basic building material of the universe, created in the Big Bang. And as we discussed above, it is also the most abundant in the universe. Oxygen is created by nuclear reactions in stars. In fact, almost all of the oxygen in space is found in the form of water or carbon monoxide. Similarly, carbon and nitrogen in space are also found in their most hydrogenated forms: methane (CH4) and ammonia (NH3). And as expected, there are huge amounts of water in the universe.
How did water form on Earth?
We know how water can be produced, but the truth is that we do not know exactly how water appeared on Earth. It is possible that water was always present in the Earth’s mantle and was gradually released to the surface by volcanoes.
Another hypothesis says that water was brought to Earth by asteroids. I discovered that there is water on Mars, on the Moon but also on other planets or satellites. It is possible that the water was brought by asteroids because there is no evidence so far showing volcanic activity on them.
In 1974, it was discovered that the Earth’s mantle contained more precious metals than estimated. These heavy elements have been attracted to the planet’s iron core since the beginning of Earth’s formation. This discovery led to the idea that asteroids hitting Earth brought a surplus of chemical elements. It is possible that these asteroids brought, in addition to precious metals, “volatile” substances such as carbon and water, which are known to exist on a type of asteroid type C (carbonic chondrite).
In 2017, a study published in the journal Nature by Mario Fischer-Godde of the Institute of Planetology at the University of Munster showed that ruthenium in the Earth’s mantle has a different isotopic composition than that found in asteroids in the solar system that reached the Earth.
It is considered that the excess of volatile elements and water were brought by asteroids (type C) or comets, but until 2017 a “genetic” link was not established or excluded to confirm this hypothesis. Such a bond can be determined using ruthenium isotopes, as Mario Fischer-Godde observed. Ruthenium isotope anomalies have shown that the element formed at a greater heliocentric distance contains larger variations of isotopes and therefore asteroids were not the primary source of volatile elements and water on Earth.
This work adds to other research that shows that water was abundant on Earth immediately after the huge impact with the planet Theia (an event estimated to have occurred 4.5 billion years ago) from which our only natural satellite would be born. For example, the oldest terrestrial minerals, such as zirconium, crystallized in magma, where it interacts with liquid water, have been discovered. These minerals are between 4.1 and 4.3 billion years old.
Moreover, just because an asteroid carries water does not mean that the water will reach and remain on Earth. On the contrary, the Earth could have lost more mass than it would have gained during the violent impacts. Although it is an unproven theory, a recent study of the crater in Sudbury, Canada, revealed that the collision vaporized most of the volatiles.
Another indication that the planet’s oceans formed very early is that there is more chlorine on Earth than we would expect. The early presence of liquid water would have given chlorine an environment in which to dissolve and thus help prevent its dissipation into space. Moreover, geochemists claim that the Earth’s oceans did not form of glacial comets because they contain different amounts of heavy hydrogen (deuterium).
All this evidence suggests that the Earth’s liquid hydrosphere comes from inside the Earth. The water was stored in the Earth’s mantle in the form of hydroxyl groups (a hydrogen atom and an oxygen atom) wlelded into magnesium silicate or other minerals. When the mantle melted, the water dissolved in the magma. As the magma rose to the surface and cooled, the pressure dropped and crystals formed, and water was released and emitted as vapors through volcanoes. Through this mechanism, water from a great depth could be released to the surface.
It is important to understand that water can be recycled back into the mantle. This means that there is a balance between the water in the oceans and that stored in the mantle of the Earth. What we do know is that the average level of the sea surface relative to the dry surface has remained relatively constant in almost 4 billion years. This suggests that a constant cycle of water that occurs and is absorbed into the mantle has significantly helped life continue throughout its history on this planet.
Abundance of water in the Universe
How abundant is water in the universe? It is very likely to exist even on the surface on all planets that have an atmosphere that contains sufficient amounts of ozone.
The sun, among other radiation, emits ultraviolet rays that can break down water molecules back into oxygen and hydrogen. But the same sun in the early solar system, scattered around gas radiation called solar winds. Some of them reached Earth and formed the Earth’s atmosphere. In the stratosphere, an oxygen molecule (O2) when it absorbs ultraviolet rays from the Sun is decomposed into 2 oxygen atoms (O + O). The two atoms are now free to react with an oxygen molecule (O2) to create an ozone molecule (O3). Thus, the Earth’s atmosphere created a protective layer that allowed water and later life to exist.
Atmosphere on other planets
If the atmosphere plays an important role in the existence of liquid water on the planet’s surface, how common are the planets that have an atmosphere? The factor that determines whether a planet has a stable atmosphere is the force of gravity. Massive planets have a strong gravity and gas molecules cannot escape into space. On the other hand, a small body like Mercury or the Moon cannot hold the gases together because the thermal velocity of the molecules is higher than gravity. But the other planets in our solar system have an atmosphere and we even have 4 gas giants that we can say are almost entirely “atmosphere”: Jupiter, Saturn, Uranus and Neptune.
The presence of water must be common in other solar systems. Many planets have been discovered in the circumstantial habitable zone (region in space defined on the basis of conditions to ensure the existence of liquid water) but we will have to wait until March 2021, when the James Webb telescope will be launched to find out whether or not we are alone in the universe.